Description
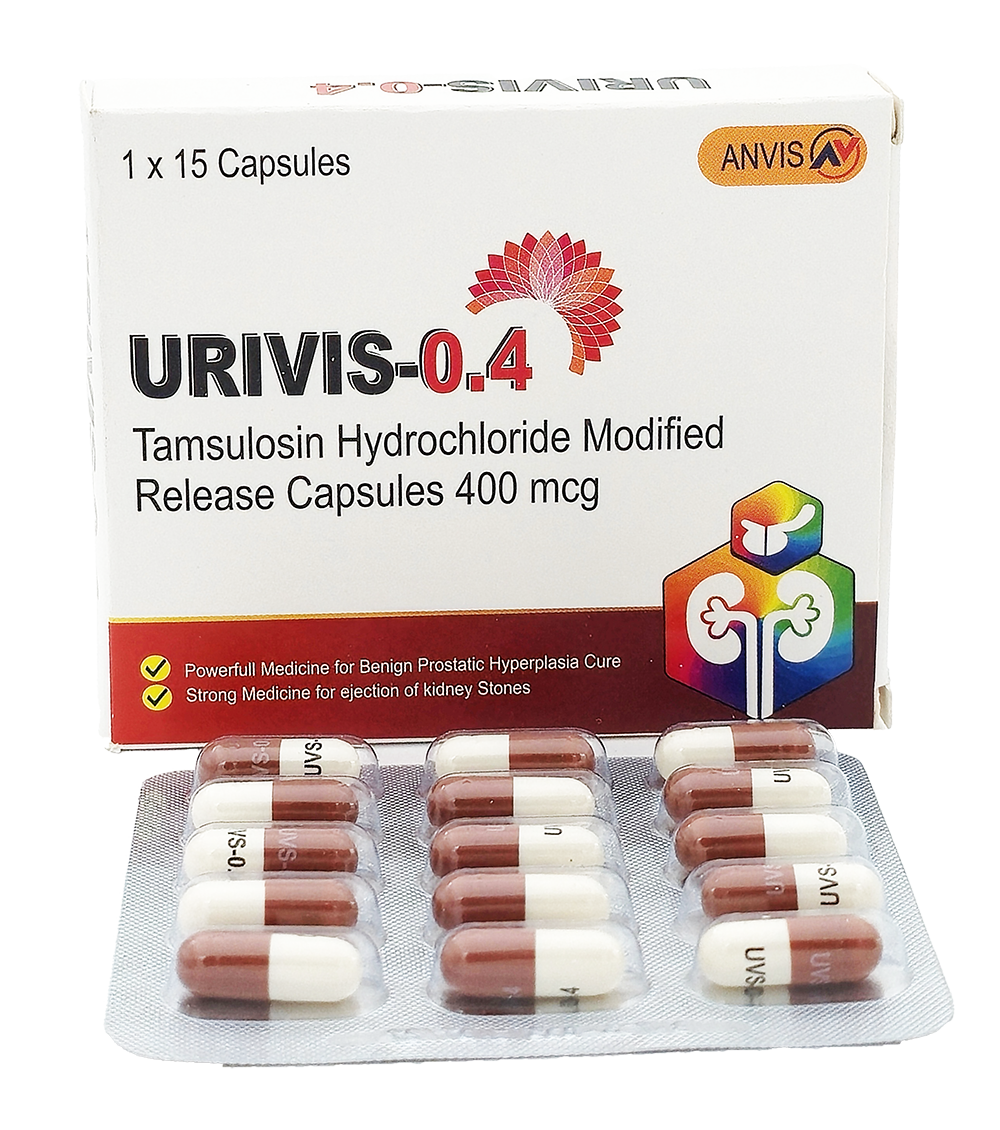
URIVIS‑0.4
is a modified-release capsule manufactured by ANVIS, containing Tamsulosin Hydrochloride 400 mcg. Each box includes 15 capsules. It is indicated for the treatment of Benign Prostatic Hyperplasia (BPH) and urinary obstruction due to kidney stones.
Pharmacological class:
- Alpha‑1 adrenergic receptor antagonist
Mechanism of action:
Tamsulosin selectively blocks alpha‑1 receptors in the smooth muscle of the prostate and bladder neck, leading to muscle relaxation and improved urine flow. It also facilitates the passage of kidney stones by relaxing ureteral smooth muscles.
Indications:
- Benign Prostatic Hyperplasia (BPH)
- Difficulty in urination due to enlarged prostate
- Ejection of kidney stones
- Lower urinary tract symptoms (LUTS)
Benefits:
- Improves urine flow and reduces urgency/frequency
- Facilitates stone passage without surgery
- Modified-release formulation for consistent plasma levels
- Well-tolerated with minimal cardiovascular effects
Packaging:
- Box of 15 modified-release capsules
- Dosage: Tamsulosin Hydrochloride 400 mcg
Safety notes:
- Should be taken after the same meal daily
- May cause dizziness or orthostatic hypotension
- Not recommended for use with strong CYP3A4 inhibitors
- Monitor renal function in patients with kidney impairment
Market positioning:
URIVIS‑0.4 offers dual utility in BPH management and kidney stone ejection, making it a strategic choice for urologists seeking non-invasive symptomatic relief. Compared to standard alpha‑blockers, its modified-release profile ensures better tolerability and patient compliance.